
This way forward to new possibilities
ABOUT XENPOZYME


XENPOZYME is the first and only disease-specific treatment for ASMD
XENPOZYME targets enzyme deficiency—the underlying cause of ASMD
XENPOZYME provides the enzyme that is deficient, helping to reduce the buildup of a substance called sphingomyelin in cells. XENPOZYME is not expected to impact symptoms related to the central nervous system.

In adults and children, XENPOZYME showed improvements across multiple organs:

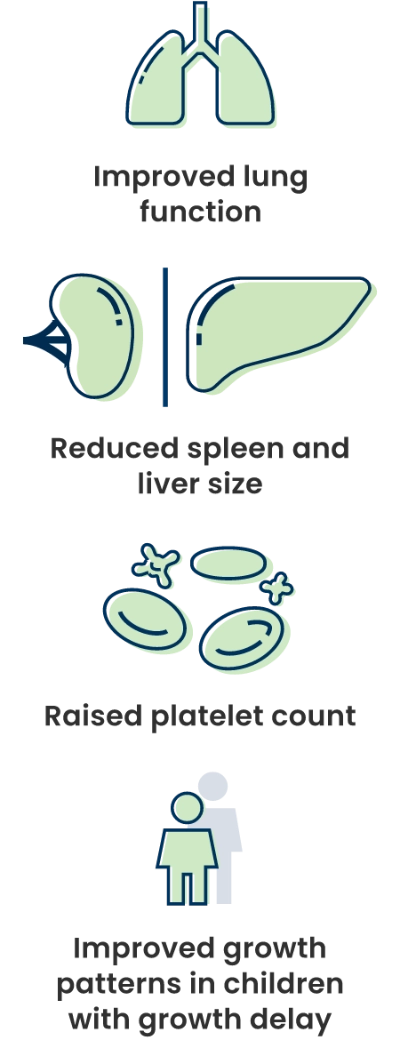
When studying how XENPOZYME worked in adults and children in the clinical trials, these were key areas:
- ASMD can impact how the lungs work. These trials studied XENPOZYME to see whether it could help the lungs bring more oxygen into the bloodstream.*
- ASMD can cause the spleen and/or liver to be larger than normal, which can cause abdominal pain and loss of appetite. These trials studied XENPOZYME to see whether it could reduce spleen and/or liver size.
- ASMD can lead to a low platelet count, which can cause easy bleeding and bruising. These trials studied XENPOZYME to see whether it could increase platelet counts.
- Some children with ASMD may experience growth delay. The trials in children studied XENPOZYME to see if it could help improve growth patterns.
*As measured by DLco.
ASM=acid sphingomyelinase; ASMD=acid sphingomyelinase deficiency; DLco=diffusing capacity of the lungs for carbon monoxide.
Discover how XENPOZYME helped adults and children with ASMD.
IMPORTANT SAFETY INFORMATION AND INDICATION
|
WARNING: SEVERE ALLERGIC REACTIONS Allergic Reactions Including Anaphylaxis |
WARNINGS AND PRECAUTIONS
Allergic Reactions (Including Anaphylaxis) and Infusion-Associated Reactions (IARs)
See Boxed WARNING for more information. Your doctor may decide to give you antihistamine, anti-fever, and/or steroid medications before your infusions. Signs of allergic reactions and infusion-associated reactions (IARs) included hives, itchy skin, skin redness, rash, swelling underneath the skin, tender bumps under the skin, and localized swelling, as well as headache, vomiting, nausea, fever, and diarrhea.
Reactions may occur during and/or after the infusion. Tell your healthcare provider right away if you experience any reactions. Your healthcare provider may slow or stop the infusion or may lower the next dose.
Elevated Transaminases Levels
XENPOZYME may be associated with elevated liver enzymes (known as transaminases) within 24 to 48 hours after infusion. Your doctor should check your liver enzyme levels with a blood test:
- within one month before starting XENPOZYME;
- within 72 hours before any infusion during the dose escalation phase, or before your next scheduled XENPOZYME infusion if you missed a dose.
Based on the results of your blood tests, your doctor may make changes to your dose or infusion schedule. Upon reaching the recommended maintenance dose, your doctor may continue to monitor your liver enzyme levels.
Risk to Unborn Babies
Starting or increasing the dose of XENPOZYME is not recommended in a pregnant female as it may cause harm (birth defects) to the developing baby. If you are pregnant or plan to become pregnant, tell your doctor right away.
If you are a female of reproductive potential, your doctor will verify your pregnancy status before you start treatment with XENPOZYME. You should use effective contraception during XENPOZYME treatment and for 14 days after your last dose if XENPOZYME is discontinued.
ADVERSE REACTIONS
- Most frequently reported adverse drug reactions in adults (incidence ≥10%) were headache, cough, diarrhea, low blood pressure, and redness in the eye.
- Most frequently reported adverse drug reactions in pediatric patients (incidence ≥20%) were fever, cough, diarrhea, runny nose, abdominal pain, vomiting, headache, hives, nausea, rash, joint pain, itchy skin, fatigue, and sore throat.
INDICATION
XENPOZYME® (olipudase alfa-rpcp) is indicated for treatment of non–central nervous system manifestations of acid sphingomyelinase deficiency (ASMD) in adult and pediatric patients.
Please see full Prescribing Information, including Boxed WARNING, for complete details.
IMPORTANT SAFETY INFORMATION AND INDICATION
|
WARNING: SEVERE ALLERGIC REACTIONS Allergic Reactions Including Anaphylaxis |
WARNINGS AND PRECAUTIONS
Allergic Reactions (Including Anaphylaxis) and Infusion-Associated Reactions (IARs)
See Boxed WARNING for more information. Your doctor may decide to give you antihistamine, anti-fever, and/or steroid medications before your infusions. Signs of allergic reactions and infusion-associated reactions (IARs) included hives, itchy skin, skin redness, rash, swelling underneath the skin, tender bumps under the skin, and localized swelling, as well as headache, vomiting, nausea, fever, and diarrhea.
Reactions may occur during and/or after the infusion. Tell your healthcare provider right away if you experience any reactions. Your healthcare provider may slow or stop the infusion or may lower the next dose.
Elevated Transaminases Levels
XENPOZYME may be associated with elevated liver enzymes (known as transaminases) within 24 to 48 hours after infusion. Your doctor should check your liver enzyme levels with a blood test:
- within one month before starting XENPOZYME;
- within 72 hours before any infusion during the dose escalation phase, or before your next scheduled XENPOZYME infusion if you missed a dose.
Based on the results of your blood tests, your doctor may make changes to your dose or infusion schedule. Upon reaching the recommended maintenance dose, your doctor may continue to monitor your liver enzyme levels.
Risk to Unborn Babies
Starting or increasing the dose of XENPOZYME is not recommended in a pregnant female as it may cause harm (birth defects) to the developing baby. If you are pregnant or plan to become pregnant, tell your doctor right away.
If you are a female of reproductive potential, your doctor will verify your pregnancy status before you start treatment with XENPOZYME. You should use effective contraception during XENPOZYME treatment and for 14 days after your last dose if XENPOZYME is discontinued.
ADVERSE REACTIONS
- Most frequently reported adverse drug reactions in adults (incidence ≥10%) were headache, cough, diarrhea, low blood pressure, and redness in the eye.
- Most frequently reported adverse drug reactions in pediatric patients (incidence ≥20%) were fever, cough, diarrhea, runny nose, abdominal pain, vomiting, headache, hives, nausea, rash, joint pain, itchy skin, fatigue, and sore throat.
INDICATION
XENPOZYME® (olipudase alfa-rpcp) is indicated for treatment of non–central nervous system manifestations of acid sphingomyelinase deficiency (ASMD) in adult and pediatric patients.
Please see full Prescribing Information, including Boxed WARNING, for complete details.


